DNA discovery from CCS gives hope for new treatments of aggressive cancer diseases
Researchers from the DNRF’s Center for Chromosome Stability (CCS) at the University of Copenhagen have discovered that our cells copy their DNA much more loosely than hitherto believed. The discovery gives new insights into DNA replication, and with this information, the researchers will examine whether it can be the foundation for entirely new cancer treatments. The study was made in collaboration with researchers from Milan and was recently published in the scientific journal Cell Reports.
Researchers have found that our bodies’ cells can both survive and multiply under significantly more stress than previously thought. The discovery gives researchers new insights into DNA replication – a copy of DNA during cell division – and may potentially be used as a foundation for a new type of treatment for cancer patients. Behind the discovery is a research team from the DNRF center CCS, including Associate Professor and group leader Luis Toledo, Ph.D. students Gijs Zonderland and Sampath Amitash Gadi, post-doc Amia Ercilla, and previous post-doc at CCS Jan Benada in collaboration with researchers from Italy.
“If we are visionaries, I would say that we might be at the birth of a whole new set of molecules that could be used in fighting cancer. If we turn the finding on its head, this novel strategy aims at exploiting an in-built weakness in cancer cells and makes them crash while they divide,” said Luis Toledo, associate professor at CCS and senior author behind the study.
Protective umbrella
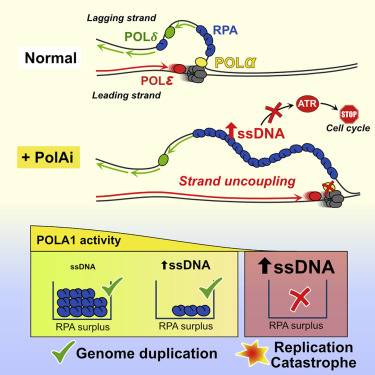
When a cell divides, the double DNA strand is opened lengthwise, and new double strands are built at each of the separated strands. Before the new halves of the double strands are made, a bit of DNA is temporally exposed in a single-stranded form.
Until now, researchers viewed large amounts of single-stranded DNA as a sign of replication stress during cell division. Now, researchers from CCS and Italy have discovered that the DNA strands open more loosely and independently of each other than expected.
The loose opening can result in the generation of large amounts of single-stranded DNA caused by natural stress and something the cells can easily tolerate, according to researchers. However, tolerance requires that the cells have enough amounts of a protective protein called RPA to cover the exposed single-stranded DNA-parts.
“We have seen that cells can duplicate their genome, even with large amounts of single-stranded DNA. They can divide and go on living healthily because they have a large excess of RPA molecules that acts like a protective umbrella. But there is a flip side of the coin. When we make the cells generate single-strand DNA faster than what they can protect, chromosomes literally shatter in hundreds of pieces, a phenomenon we call replication catastrophe. We always thought that we could use this, for instance, to kill cancer cells,” said Amaia Ercilla, a post-doc at CCS and leading author behind the study.
Yet, it is challenging and time-consuming for the researchers to cause a replication catastrophe in the cells by emptying the cells’ RPA reserves. Until now, it took around an hour to use RPA reserves with different types of chemotherapy to increase the amount of single-stranded DNA and thus provoke a replication catastrophe and cell death.
When the researchers began inhibiting the essential gene DNA polymerase alpha, or POLA1, which initiates DNA replication during cell division, something happened: after just five minutes, the researchers were able to kill the cells – a method the researchers hope can be used to kill cancer cells faster and more efficiently.
“Although no new DNA can be made when we inhibit POLA1, the DNA unzippers keep advancing and generate single-stranded DNA at very high speed. All cells can be sensitive to POLA1 inhibitors, including cancer cells, and we might speculate that the strategy could be especially useful against very aggressive forms of cancer that proliferate at a high pace,” said Toledo.
