CNG researchers show how electric fields can be used to control chemical bonds
A new experimental technique has made it possible to use electric fields to measure the electrical resistance and to control the strength of the individual chemical bond. The theoretical and experimental study, a collaboration between the Center of Excellence CNG at DTU and the Ilmenau Technical University in Germany, may lead to new applications and opportunities in nanotechnology. The study has been published in the scientific journal Physical Review Letters.
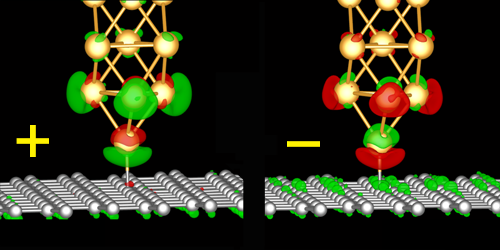
A theoretical and experimental study has uncovered a new technique that uses electric fields while measuring the electrical resistance and controlling the strength of the individual chemical bond. The study, which is a collaboration between the Center of Excellence CNG at the Technical University of Denmark and the Ilmenau Technical University in Germany, has been published in the scientific journal Physical Review Letters.
“The presented method represents a controlled approach to the manipulation of matter and now belongs to the toolbox of nano-engineers. The manipulation of matter atom by atom has become ubiquitous. The results presented in our work rely on exploiting the short-range bond force in a single-atom chemical bond whose strength depends on the polarity of this bond,” said Professor Jörg Kröger from the Ilmenau Technical University in Germany, who is last author on the study.
New opportunities in nanotechnology
The method used a single gold atom at the tip of an atomic force microscope and a carbon atom, which was then moved by the graphene, proving that it can be controlled by the field. The graphene was controlled by the direction of the field, which made it either more or less “sticky,” depending on the direction in the field. CNG researchers have been able to explain this with atomic calculations.
“Theoretical ideas of influencing chemical bonds by an external electric field are longstanding, but detailed experiments about the individual chemical bond have been missing. The experimental realization was therefore a strong motivation to simulate the findings and understand the system in detail to see how the theory fits,” said Professor Mads Brandbyge from CNG, who is co-author on the study.
